- Home >
- Applications >
- Biofilm >
- Biofilm Extended
Biofilm Extended
Complex biofilms
Due to their inherent complexity, biofilms present a significant challenge in scientific research. These intricate structures are home to countless coexistent subpopulations, each playing different roles. The difficulty lies in understanding who is doing what within this dense and diverse environment. Traditional methods fall short, often providing a fragmented view that misses the full picture of these cellular communities.
To truly grasp the nature of biofilms, we have to measure the entire population holistically. Researchers need a way to observe all cellular activities, interactions, and subpopulations in a unified manner. This is where the Symcel calScreener™ comes in. With its innovative technology, the calScreener™ measures the metabolic activity of all cells, regardless of their position within the biofilm. By capturing all processes, interactions, and subpopulations simultaneously, it offers a comprehensive view that was previously unattainable.
The calScreener™ makes it easy
The challenge intensifies when studying biofilms that involve multiple species. The interactions between different species and strains add layers of complexity, making it difficult to discern the ecological dynamics at play. Understanding these multispecies interactions is crucial, yet the intricate ecology of biofilm niches often impairs our ability to fully comprehend the contributions of each group.
The calScreener™ provides a powerful solution to this problem as well. By measuring the activity of all cells in the sample, it allows researchers to detect and analyze the competition, synergy, and interactions between different species and strains within the biofilm. This capability enables a deeper understanding of biofilm ecology, shedding light on the complex interplay of different microbial groups.

Dormant subpopulations
One of the most pressing challenges in biofilm research is the detection of dormant subpopulations. These cells, often hidden deep within the biofilm, are particularly elusive. Traditional methods struggle to detect these non-growing, dormant cells, yet they are among the most critical to understand, especially when it comes to measuring antimicrobial tolerance. These deep-seated cells are often the key to understanding how biofilms survive in hostile environments, but their inaccessibility makes them difficult to study.
To address this challenge, we need the capability to measure the survival of all subpopulations within a biofilm, including those that are dormant or non-growing and those buried deep within the structure. It’s crucial to have a method that doesn’t just scratch the surface but dives deep into the biofilm to capture the full spectrum of cellular activity.
The Symcel calScreener™ offers a unique solution to this problem. It measures the metabolic activity of all cells, regardless of their location within the biofilm. Whether the cells are active or dormant, on the surface or deep within the biofilm, the calScreener™ captures all processes, interactions, and subpopulations. This comprehensive approach ensures that no part of the biofilm goes unnoticed, providing researchers with the critical insights needed to understand antimicrobial tolerance and survival mechanisms within these complex communities.
With the calScreener™, you gain the ability to measure what was previously undetectable, opening new avenues for research and deeper understanding of biofilm resilience.
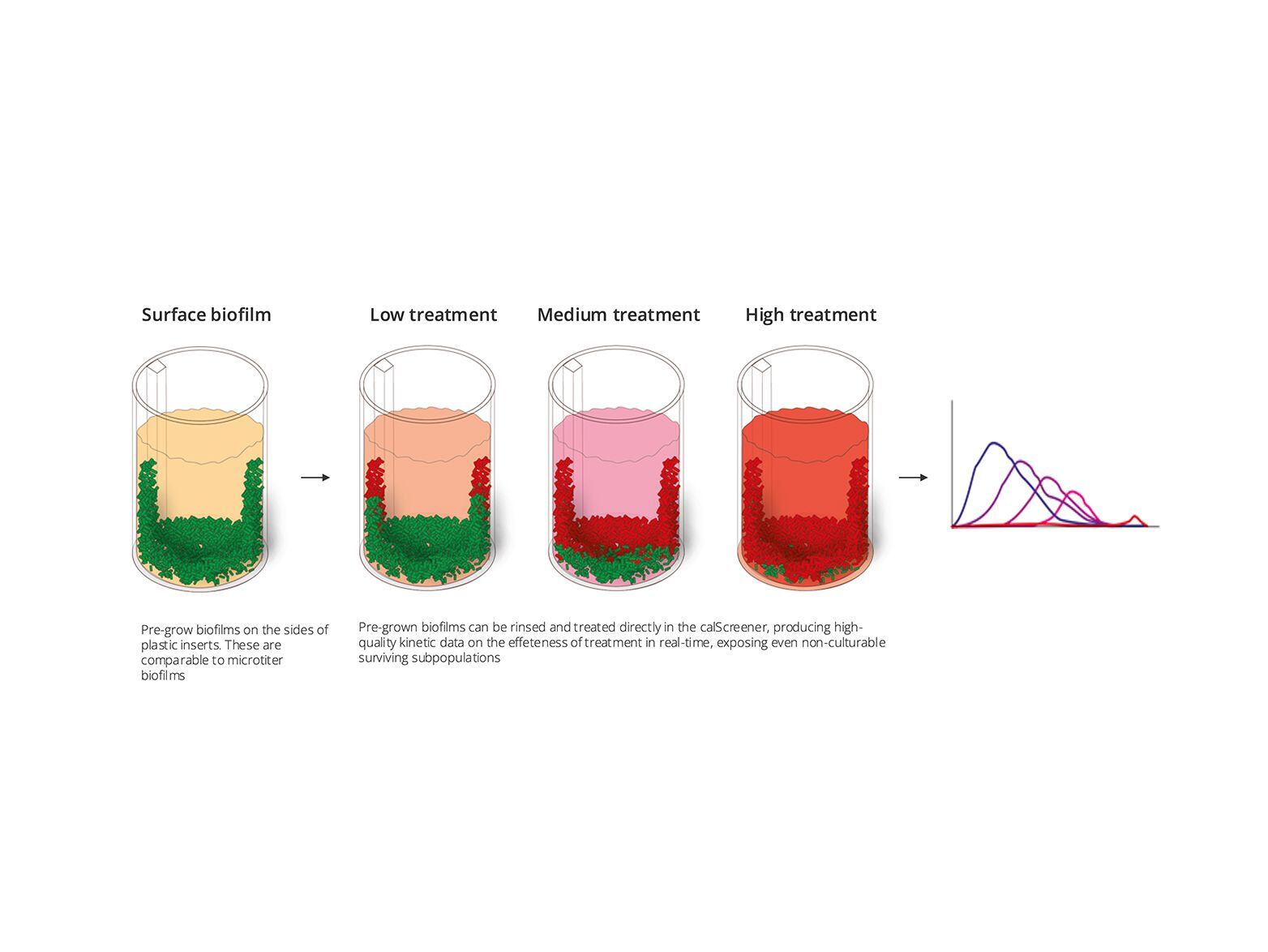
Clinically and environmentally relevant model systems
One of the persistent challenges is the relevance of traditional biofilm models. These models often fall short of accurately representing clinical or environmental conditions. While it’s possible to create models that closely mimic in vivo conditions, they often become complex and difficult to interpret. Bacteria in these models frequently form biofilm aggregates, which can lead to underrepresentation of data when using traditional colony-forming unit (CFU) plate counts. This limitation hinders our ability to gain a true understanding of biofilm behavior in real-world scenarios.
To overcome these obstacles, researchers need a technology that can penetrate complex matrices and see deep inside tissues, pus, or other intricate models. The ability to detect and measure biofilm activity in situ, without disturbing the natural environment of the sample, is crucial for obtaining accurate and clinically relevant data.
The Symcel calScreener™ provides the solution to this problem. It lets you measure and detect all cells, including non-growing ones, directly within their natural environment. Whether dealing with complex and messy model systems or real clinical samples, the calScreener™ lewts you observe biofilms in their true state, without the need to remove them from their original context. This approach ensures that the data collected is both relevant and reflects real-world conditions, providing valuable insights into biofilm behavior and antimicrobial responses.
With the calScreener™, you can finally bridge the gap between traditional models and clinically or environmentally relevant scenarios, paving the way for more accurate and impactful biofilm research.

Quantify biofilms without disruption
Most traditional biofilm techniques require the researcher to remove the cells from the biofilm to quantify them with simple plating and CFU count. It can be a significant challenge to remove the bacteria from the biofilm structure to count them. Once a biofilm is established, however, it becomes nearly impossible to disturb it enough to create a perfect, homogeneous single-celled suspension. Even when cells are successfully removed, they may enter a state where they are viable but not culturable, meaning they won’t grow on plates for a traditional colony count. This makes it extremely difficult to accurately measure the true population of viable cells within a biofilm.
You need a way to detect all viable cells within the biofilm, including those that are dormant, non-growing, or unable to grow on agar plates. Additionally, it’s essential to have a method for detecting these bacterial cells in situ, without the need to remove them from the biofilm. This allows more accurate measurements, reflecting the true state of the biofilm without disturbing its structure.
With the calScreener, you can overcome the limitations of traditional methods and achieve a more accurate and comprehensive understanding of biofilm populations.
Get more and deeper data.
When studying biofilms, one of the most significant challenges is determining whether cells are resistant or merely tolerant to a given treatment. Traditional analysis methods often fall short by providing only binary results—cells are classified as either dead or alive, or rather, if the cells are able to form a colony on an agar plate or not. This limited approach fails to capture the nuanced responses that occur within biofilms, where different subpopulations may react in varied ways to the same treatment.
To gain a deeper understanding, researchers need kinetic data that reveals treatments’mode of action – whether they are bacteriostatic (inhibiting growth) or bactericidal (killing the cells). This detailed information is crucial for developing effective strategies to combat biofilm-related infections and for tailoring treatments to achieve the desired outcome.
The Symcel calScreener provides non-binary kinetic data on cell survival, the effects of treatments, and the survival of different subpopulations within the biofilm. By capturing these detailed responses, you can distinguish between resistance and tolerance, track the efficacy of treatments over time, and gain insights into the behavior of biofilms under various conditions.
Move beyond simple dead-or-alive assessments and gain a comprehensive understanding of how biofilms respond to treatments, paving the way for more precise and effective therapeutic approaches. The calScreener™ is your answer.