- Home >
- Clinical Application Research Extended
Clinical Application Research Extended
Next-gen tech for diagnosing complicated infections
Periprosthetic Joint Infection (PJI) is one of the most daunting complications in orthopedic surgery, with profound implications for patient health and significant financial burdens on the healthcare system. These infections occur when bacteria or fungi colonize the space around a joint prosthesis and the soft tissue, leading to severe complications that often require complex and costly treatment strategies.
Diagnosing PJIs is notoriously challenging due to their varied and often ambiguous clinical presentations, which can easily be mistaken for other issues, such as aseptic implant loosening. Symptoms range from acute inflammation to chronic pain and stiffness, making early detection difficult. Additionally, the diverse range of microorganisms that can cause PJI—including antibiotic-resistant strains and those forming biofilms—further complicates diagnosis and treatment.
Current methods are limited
Traditional methods for diagnosing PJIs, primarily culture-based techniques, have long been considered the gold standard. However, these methods come with significant limitations. Culturing synovial fluid or tissue samples to isolate and identify the causative organisms can take several days to weeks, delaying diagnosis and appropriate treatment. Moreover, these methods are prone to producing false-negative results, especially in cases where the microorganisms are difficult to culture, or the patient has been pre-treated with antibiotics. Up to 20% of PJIs go undiagnosed with these traditional methods, leaving patients vulnerable to prolonged and ineffective treatment.
Molecular diagnostics have emerged as a valuable tool in PJI detection, offering faster and more sensitive alternatives to culture-based methods. Techniques such as 16S rRNA sequencing and multiplex PCR can identify bacterial DNA directly from clinical samples, which is useful for detecting slow-growing or previously treated organisms. However, molecular methods are not without their drawbacks. They can detect DNA from non-viable organisms, leading to false-positive results and unnecessary treatments. Furthermore, these methods are often expensive, technically complex, and require significant expertise to interpret, limiting their accessibility in many healthcare settings.
We need a method capable of providing reliable results in a much shorter timeframe, facilitating timely intervention and treatment. It should also be sensitive enough to detect even low levels of bacterial activity and versatile enough to identify biofilms and antibiotic-resistant strains. Importantly, this method should support antibiotic stewardship by enabling precise, tailored antimicrobial therapy, thereby reducing the risk of antibiotic resistance development.
The new gold standard
Isothermal microcalorimetry (IMC), or biocalorimetry, is a groundbreaking solution to these challenges. It works by measuring the heat produced by the metabolic activity of microorganisms in real-time, offering several key advantages over existing techniques. Unlike culture-based methods, IMC can deliver results within hours to a few days, significantly speeding up the diagnostic process. Its high sensitivity lets your detect low levels of bacterial activity, making it particularly effective in identifying slow-growing or fastidious organisms that other methods might miss. Additionally, IMC is uniquely capable of detecting the metabolic activity of bacteria within biofilms—a common and challenging feature of PJIs. This capability is crucial for early detection and effective treatment planning.
One of the most significant benefits of IMC is its non-specific growth requirement, meaning it does not rely on prior knowledge of the infecting organism. This reduces the biases inherent in designing specific molecular probes and allows for a more comprehensive analysis of the sample. Moreover, IMC’s non-destructive nature means that other diagnostic tests can be performed on the same sample, providing a more holistic diagnostic approach.
Beyond diagnosis, IMC can be used for rapid antimicrobial susceptibility testing, offering real-time feedback on the effectiveness of antibiotic treatments. This lets you customize antibiotic therapy to the specific needs of the patient, supporting a more personalized medicinal approach and contributing to antibiotic stewardship efforts.
Speed and Detection
The use of implants is increasingly common, with projections of over 20 million implant surgeries annually by 2030. While these implants have revolutionized patient care, they also come with a significant risk—implant-related infections. Prosthetic joint infections (PJI) are a particular concern due to their severity and the challenges associated with diagnosing them. Traditional culturing methods are time-consuming and not always effective. This is especially true for infections involving biofilms, where bacteria can enter a dormant state, making them difficult to detect using conventional techniques. Moreover, when these microbes are removed from the biofilm and cultured on agar plates, they may not survive, further complicating the diagnosis. As the prevalence of antibiotic-resistant strains continues to rise, the limitations of these traditional methods become even more pronounced, leading to delays in diagnosis and treatment that can significantly impact patient outcomes.
We need a diagnostic approach that is not only faster but also more sensitive to the unique difficulties presented by implant-related infections. Specifically, we need a method that can quickly and accurately detect infections, even in cases involving biofilms or antibiotic-resistant bacteria.
Recent research, including a study by Cichos, K. H., et al. (2022) titled “Isothermal Microcalorimetry Improves the Time to Diagnosis of Fracture-related Infection Compared with Conventional Tissue Cultures,” highlights IMC’s potential in this context. The study demonstrates that the calScreener™, an IMC-based tool, can detect infections with a sensitivity and specificity comparable to traditional culture methods but in a fraction of the time. The median detection time with the calScreener was just 2 hours, compared to an average of 51 hours using conventional methods. Even in patients with a history of chronic antibiotic use—a group for whom traditional methods often struggle—the calScreener maintained its rapid detection capabilities without sacrificing accuracy.
These findings suggest that the calScreener could substantially advance the diagnosis of implant-related infections. By offering quicker and more precise detection, this technology has the potential to significantly improve patient outcomes through earlier intervention and more targeted treatment. Furthermore, the ability of IMC to detect the metabolic activity of bacteria within biofilms opens new possibilities for addressing one of the most challenging aspects of PJI management. While further research and clinical validation are necessary, the calScreener’s performance in early studies suggests that it could play a crucial role in the future of infection diagnostics, particularly in the complex and growing field of implant-related infections.
Rapid Identification
Identifying the specific microbes responsible for Periprosthetic Joint Infections (PJIs) presents a significant challenge, especially when the complex nature of the samples involved hinders traditional diagnostic methods. When microbial activity is detected or suspected in PJI cases, pinpointing the exact cause of the infection can be difficult. Molecular techniques, while advanced, can be challenging due to the high amount of host material present in samples like biopsies. This excess host material can interfere with the sensitivity and specificity of these methods, making it challenging to achieve accurate identification.
Other promising technologies, such as mass spectrometry, offer precise microbial identification but come with their own set of limitations. Mass spectrometry typically requires pure, single-species colonies grown on culture plates to generate accurate results. However, in the context of PJIs, growing these colonies can be particularly challenging due to the fastidious or hard-to-culture nature of some infecting organisms. The process of waiting for these colonies to grow not only delays diagnosis but also increases the risk of complications for the patient. These delays and difficulties underscore the need for a more efficient and comprehensive diagnostic approach that can overcome these barriers and provide reliable identification of infection-causing organisms.
The need for a new technology that can detect and identify these complex infections on a single platform is clear. Such a tool would need to integrate both detection and identification capabilities, allowing for the rapid and accurate determination of the microbial species involved in PJIs. This would eliminate the need for multiple testing platforms and reduce the time required for diagnosis, leading to quicker, more effective treatment.
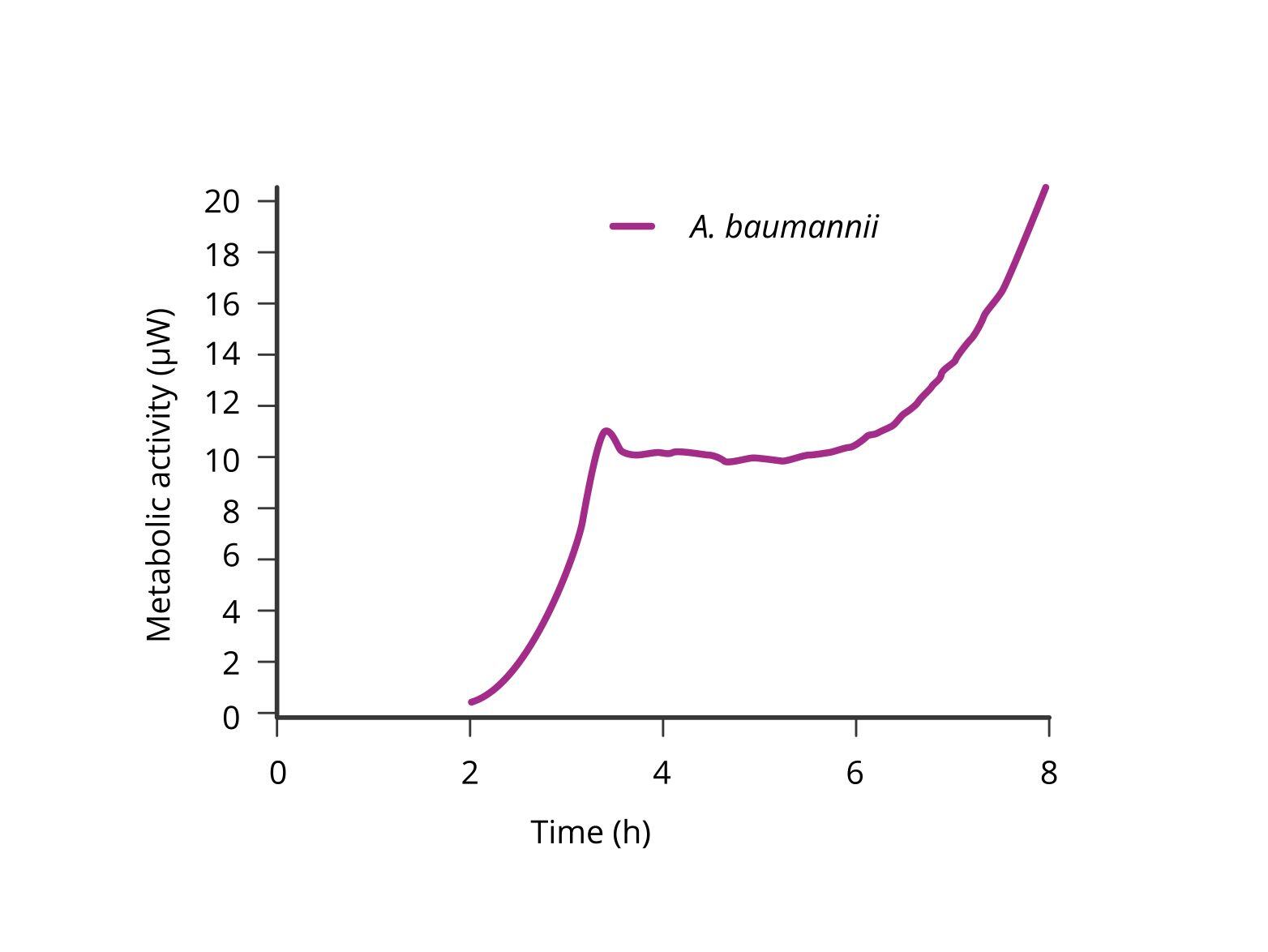
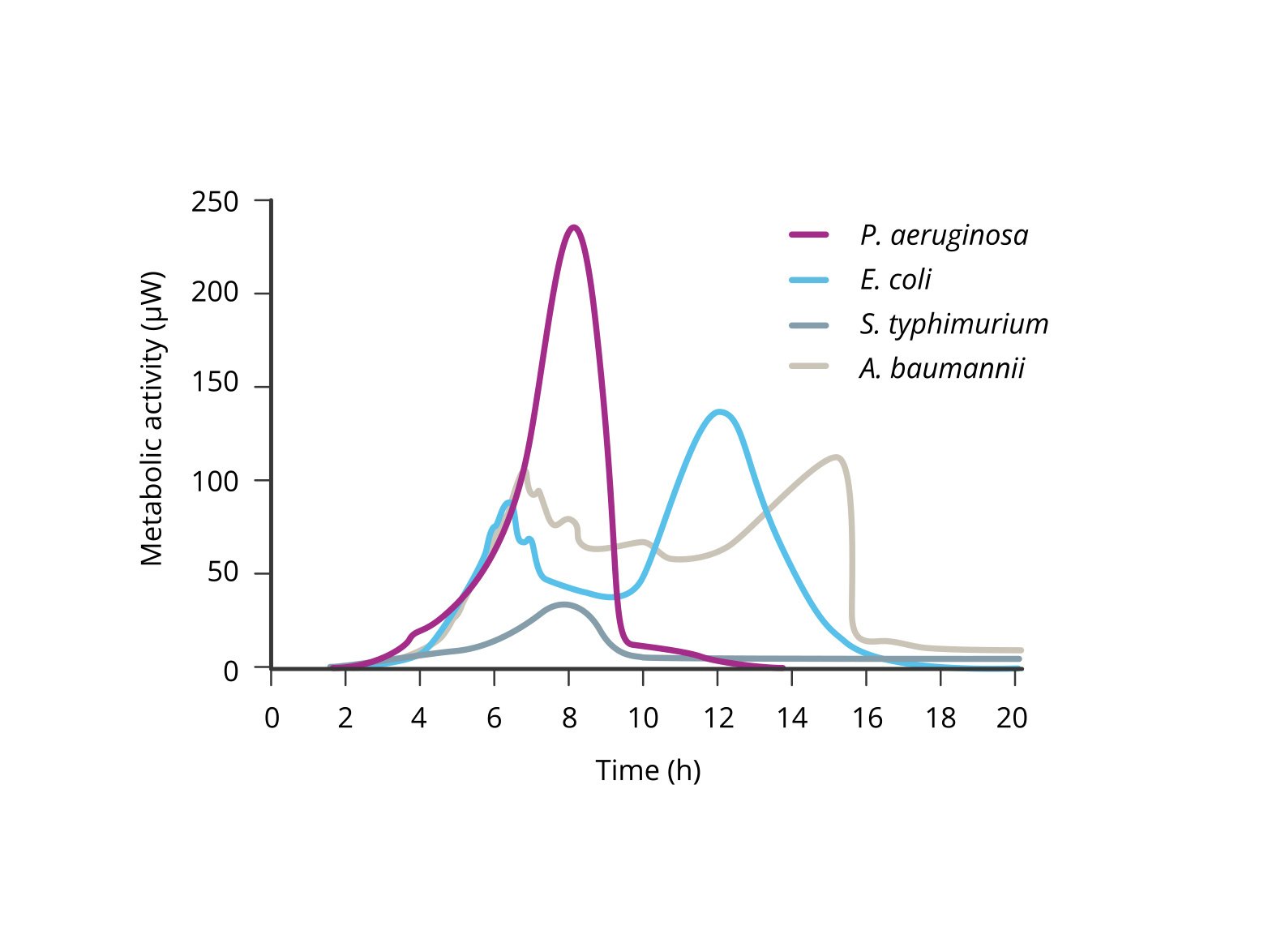
Isothermal microcalorimetry (IMC), as utilized by the calScreener, presents a groundbreaking solution to this problem. While IMC is already known for its rapid detection capabilities, peer-reviewed research has shown that the calScreener excels in accurate microbial identification as well. The calScreener translates metabolic readouts into time-trace diagrams, effectively capturing a unique “fingerprint” of the metabolic activity of different microbial species. This metabolic profile can then be used in research studies to precisely identify the organisms present in a clinical sample.
The ability to accurately identify infection-causing microbes using their metabolic fingerprints represents a significant advancement over traditional methods. Unlike mass spectrometry, which requires isolated colonies, the calScreener can work directly with the complex mixtures found in clinical samples, bypassing the need for lengthy culture processes. This not only speeds up the identification process but also increases the accuracy and reliability of the diagnosis. In an era where rapid and precise identification of pathogens is critical for effective treatment, the calScreener offers a robust and efficient solution. It simplifies the diagnostic process by providing both detection and identification on a single platform.
Fast Antimicrobial Susceptibility Testing (AST)
In the realm of infection diagnostics, one of the most significant challenges lies not only in identifying the pathogen responsible but also in determining which antibiotics will effectively combat it. Traditionally, this process involves multiple steps, beginning with the detection and isolation of the pathogen, followed by an antimicrobial susceptibility test (AST). These tests, which are often based on methods like disk diffusion assays, EUCAST, or CLSI, are critical for selecting the right antibiotic. However, they come with a major drawback: time. These methods require the pathogen to be successfully grown on culture plates, which can take days. During this waiting period, patients are typically prescribed broad-spectrum antibiotics as a precaution, a practice that can lead to prolonged discomfort for the patient and the potential development of antibiotic resistance, among others.
This conventional approach, while effective, is slow and labor-intensive. The delay in identifying the appropriate, targeted treatment means that patients are not receiving the most effective therapy as quickly as they could be. The reliance on broad-spectrum antibiotics, while necessary in some cases, is far from ideal. What is needed is a technology that can streamline this process, integrating the detection of the pathogen with the identification of its antibiotic susceptibilities, all within a much shorter timeframe. Such a technology would revolutionize the way infections are treated, allowing for quicker, more precise interventions that directly target the pathogen.
Enter the calScreener™. The calScreener™ doesn’t just identify the presence of a pathogen; it simultaneously assesses which antibiotics will be most effective against it, providing critical information in a fraction of the time required by traditional methods. The calScreener™ can deliver results for single antibiotic testing in as few as two hours. For more complex, combination therapies, results are typically available within six hours. This rapid turnaround is achieved by analyzing the metabolic activity of the pathogens, offering insights not just into whether a microbe is present, but also into how it responds to specific antibiotics.
What makes the calScreener™ truly revolutionary is its ability to distinguish between antibiotics that kill the bacteria and those that merely suppress its growth. This distinction is crucial for tailoring the most effective treatment. Independent research, including studies conducted by the University of Copenhagen, has demonstrated the calScreener’s superior predictive capabilities, showing that it can more accurately determine the best course of treatment compared to traditional methods.
In practical terms, the calScreener offers a comprehensive solution that allows for real-time adjustments to a patient’s treatment plan. By rapidly identifying both the pathogen and the most effective antibiotics, it helps clinicians move away from the blanket use of broad-spectrum antibiotics, instead focusing on targeted therapies that improve patient outcomes. This is not just a diagnostic tool; it is a critical advancement in the fight against infections, providing a faster, more accurate, and more effective approach to managing infectious diseases.
In a world where every hour can make a difference in patient outcomes, the calScreener represents a significant leap forward, offering a method to detect, identify, and treat infections with unprecedented speed and precision.
